Understanding mitophagy and its role in longevity
Mitophagy is an important quality control process in our cells. Learn about the role of mitophagy and how it is related to longevity.

January 23, 2023
9 min read
Our body is an energy-consuming machine. Every second of the day, even at rest, our cells need a constant supply of energy to keep our heart beating, our lungs breathing, and our body functioning.
The problem is that our energy-producing structures - the mitochondria - are highly susceptible to damage, and over time their ability to function efficiently declines.
Since properly functioning mitochondria are essential for life, our cells have evolved a process to keep them healthy. This process is known as the mitophagy pathway. Read on to learn the role of mitophagy in longevity and the known mitophagy triggers.
The role of mitophagy
Inside almost every one of our cells lives tiny energy-producing factories called mitochondria. They turn the food we eat and the air we breathe into a form of energy called adenosine triphosphate (ATP), which we need to live. Over time, our mitochondria become dysfunctional, and as these faulty mitochondria collect in the cell, they can cause significant damage. In fact, mitochondrial decline is one of the hallmarks of the aging process, and it has been associated with a whole host of health issues, including the development of heart disease, neurodegenerative disorders, and even some forms of cancer.
To keep our mitochondria healthy, our bodies have developed an innate quality control mechanism called mitophagy. Through this process, dysfunctional mitochondria are selectively targeted for removal from the cell, limiting the negative impact that they might have.
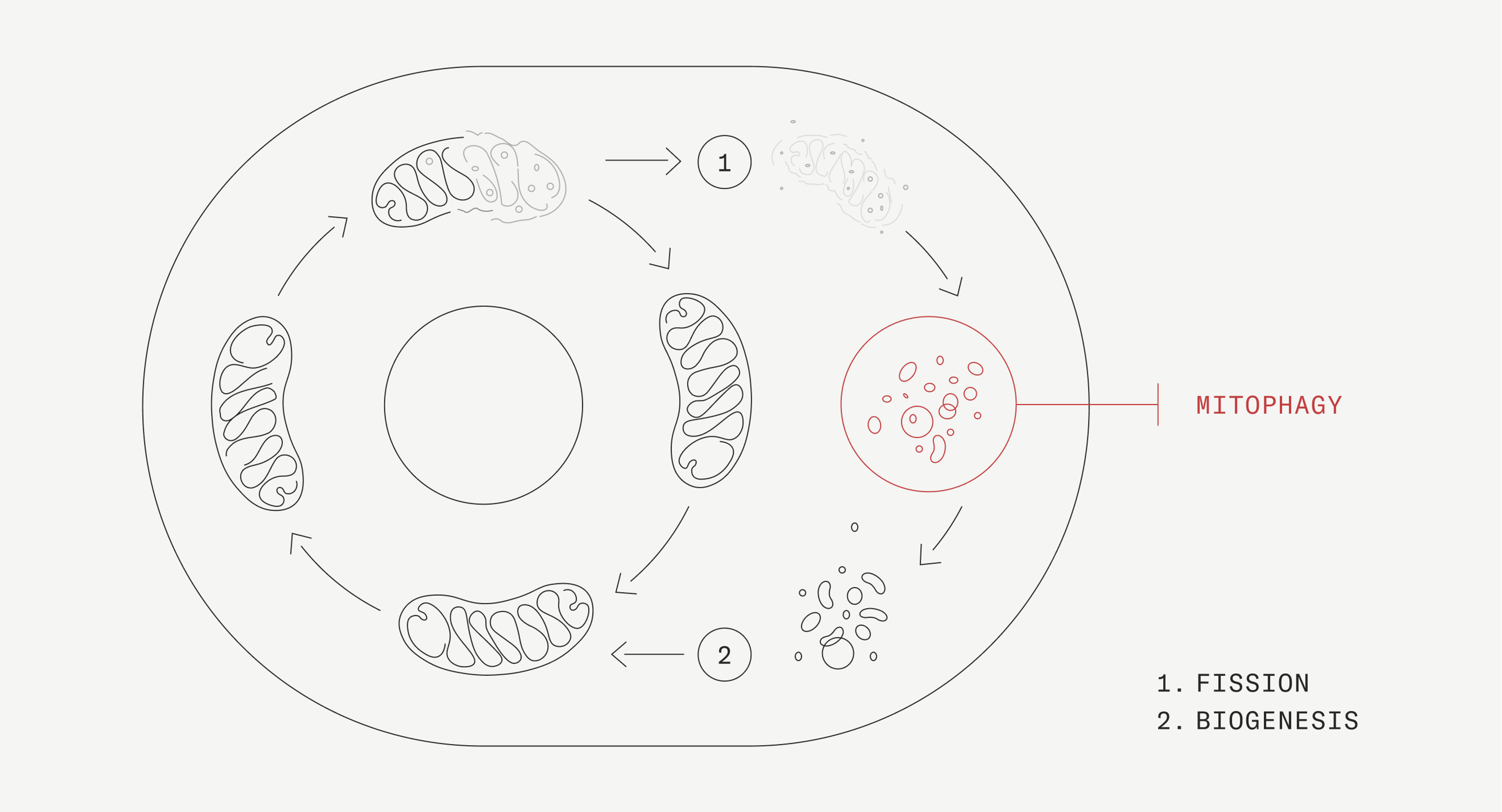
What is mitophagy?
Mitophagy is a form of autophagy where your body selectively breaks down defective mitochondria and recycles them into new, more efficient ones.
Mitophagy occurs inside the cell and only on the mitochondria. Both autophagy and mitophagy can be viewed as a form of biological cleaning and recycling. Old and damaged cellular components are cleaned out and recycled into newer, healthier cells and organelles.
Cells activate several players to trigger mitophagy when it senses that mitochondria are getting old or damaged. We can enhance this cellular activity using external inducers such as fasting, exercise, and Urolithin A.
The mitochondrial mitophagy pathway is critical to improving the quality of the pool of mitochondria in the cell, and it is tightly linked to the creation of new mitochondria, a process called mitochondrial biogenesis. This coordinated mitophagy and biogenesis process leads to improved mitochondrial function.
Mitophagy in aging
Scientists studying longevity have identified biological hallmarks of aging. These hallmarks are time-dependent processes that happen on the cellular level, and they can be either accelerated or halted by varying mechanisms.4 Importantly, a hallmark of aging is a biological process associated with aging that can be slowed down with interventions that target that particular process.
For over a decade, mitochondrial decline has been recognized as one of the hallmarks of aging. Now, in a new publication, “Hallmarks of Aging: An Expanding Universe,” the authors indicate that specifically mitophagy is also considered a hallmark of aging.
Mitophagy is related to longevity. As we age the mitochondria lose their ability to function efficiently, and we start to see abnormal mitochondrial shapes and disruption of the inner mitochondrial structures that host the energy-producing complexes. As dysfunctional mitochondria accumulate in the cell two things happen: they are less efficient in producing energy, and they can leak reactive oxygen species and mitochondrial DNA which can trigger inflammation.
Studies suggest that as we age, the clearance of dysfunctional mitochondria via mitophagy, is dysregulated, meaning that the cell is less able to clear out dysfunctional mitochondria. Without an optimized quality control mechanism such as mitophagy, cellular damage can accelerate.
In animal studies, life span extension has been seen when the genes that regulate mitophagy are expressed, demonstrating that mitophagy and longevity are related. Furthermore, impaired mitophagy is seen in several age-related diseases including Parkinson's and Alzheimer's disease, heart disease, and cancer, suggesting that interventions that target mitophagy may play a role in disease prevention and treatment.
Finding ways to induce mitophagy may be an option to help slow down the aging process.
How to increase mitophagy
Since there is a clear link between mitophagy and longevity, strategies to increase mitophagy are being researched. Clinical research has demonstrated the following ways to enhance mitophagy.

Fasting and calorie restriction. It has been known for some time that fasting and calorie restriction can induce autophagy, but recently it has been demonstrated that these behaviors may also beneficially impact the signaling pathways that stimulate mitophagy. More studies are needed to determine what type of fasting and calorie restriction, and for how long, is considered the most beneficial to induce mitophagy.

Exercise. Exercise is one of the best things you can do for health and longevity. One of the many ways that exercise promotes longevity is through its role in improving mitochondrial function and inducing mitophagy. It is thought that exercise triggers mitophagy, in part, by energetically stressing the mitochondria and by increasing the expression of proteins involved in the process. Since different types of exercise can trigger different pathways of protein expression, a well-rounded exercise program that includes strength, aerobic, and endurance training should be adopted to increase mitophagy.
Urolithin A. Urolithin A (UA) is a postbiotic compound made in our gut from dietary precursors found in pomegranates, berries, and nuts, and it is the only clinically proven substance known to trigger mitophagy.
Other mitophagy inducers. Molecules such as spermidine and NAD+ precursors such as nicotinamide, nicotinamide mononucleotide (NMN), and nicotinamide riboside (NR) have been shown to induce mitophagy in preclinical trials. To date, no clinical trials have yet demonstrated their ability to stimulate mitophagy in humans.
How does Urolithin A induce mitophagy?
There are several mitophagy pathways that UA can activate that include both PINK1–Parkin dependent and independent pathways. PINK1, short for PTEN-induced kinase 1, is an enzyme that activates the protein Parkin, initiating a cascade of events that ultimately leads to the breakdown and clearance of dysfunctional mitochondria. UA administration can trigger this pathway by stabilizing PINK1 and recruiting Parkin.
UA can also trigger PINK1-Parkin independent pathway by increasing levels of other mitochondrial proteins that promote mitophagy.
What do mitochondrial supplements do?
Mitochondrial supplements can help to support the health of the mitochondria in different ways. Nutrients, including CoQ10 and L-carnitine, support the mitochondria’s ability to generate energy. Other nutrients like resveratrol and NAD+ stimulate mitochondrial biogenesis (the creation of new mitochondria). In humans, Urolithin A is the only nutrient proven to optimize mitophagy and clear out damaged mitochondria in clinical trials.
Final thoughts
Mitophagy is a cellular quality control pathway that clears dysfunctional mitochondria from the cells and allows them to be recycled into new, more efficient mitochondria. Unfortunately, as we age, the mitophagy process is impaired, leading to the accumulation of damaged mitochondria inside the cell.
Mitochondrial decline and the accumulation of dysfunctional mitochondria contribute to the aging process and the development of many age-related chronic conditions. Therefore, inducing mitophagy may be a promising strategy to counteract age-related decline in health.
Fasting, calorie restriction, exercise, and Urolithin A are known to trigger mitophagy and, through this mechanism, may play a role in improving health and increasing lifespan. Many people struggle to maintain dietary and exercise interventions in the long term, so supplements like Mitopure may offer a unique way to optimize health.
Authors
Jennifer Scheinman
Registered Dietitian Nutritionist